What Will Cause Piston to Move Away From Base and Decrease Pressure of Helium
Learning Outcomes
- Identify the mathematical relationships betwixt the various properties of gases
- Use the platonic gas constabulary, and related gas laws, to compute the values of various gas backdrop under specified weather condition
During the seventeenth and especially eighteenth centuries, driven both by a desire to understand nature and a quest to make balloons in which they could fly (Figure 1), a number of scientists established the relationships between the macroscopic concrete properties of gases, that is, pressure, volume, temperature, and amount of gas. Although their measurements were non precise past today's standards, they were able to make up one's mind the mathematical relationships between pairs of these variables (e.chiliad., pressure and temperature, pressure and volume) that concur for an ideal gas—a hypothetical construct that real gases judge under sure conditions. Eventually, these individual laws were combined into a unmarried equation—the platonic gas law—that relates gas quantities for gases and is quite accurate for low pressures and moderate temperatures. We will consider the key developments in individual relationships (for pedagogical reasons non quite in historical order), then put them together in the platonic gas law.

Figure 1. In 1783, the first (a) hydrogen-filled airship flying, (b) manned hot air balloon flight, and (c) manned hydrogen-filled balloon flying occurred. When the hydrogen-filled balloon depicted in (a) landed, the frightened villagers of Gonesse reportedly destroyed it with pitchforks and knives. The launch of the latter was reportedly viewed by 400,000 people in Paris.
Pressure level and Temperature: Amontons'due south Law
Imagine filling a rigid container attached to a pressure gauge with gas and so sealing the container so that no gas may escape. If the container is cooled, the gas inside besides gets colder and its force per unit area is observed to decrease. Since the container is rigid and tightly sealed, both the volume and number of moles of gas remain constant. If we rut the sphere, the gas inside gets hotter (Figure two) and the pressure increases.

Figure 2. The upshot of temperature on gas pressure: When the hot plate is off, the pressure of the gas in the sphere is relatively low. Every bit the gas is heated, the pressure of the gas in the sphere increases.
This relationship between temperature and pressure level is observed for whatsoever sample of gas bars to a abiding volume. An example of experimental pressure level-temperature data is shown for a sample of air under these conditions in Effigy 3. We observe that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then P and T are directly proportional (again, when book and moles of gas are held abiding); if the temperature on the kelvin scale increases by a certain cistron, the gas pressure increases past the aforementioned factor.
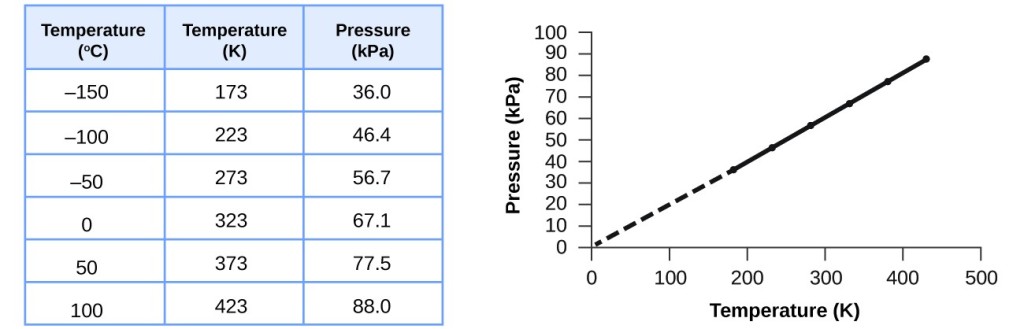
Figure 3. For a abiding volume and amount of air, the pressure and temperature are directly proportional, provided the temperature is in kelvin. (Measurements cannot exist made at lower temperatures considering of the condensation of the gas.) When this line is extrapolated to lower pressures, it reaches a pressure of 0 at –273 °C, which is 0 on the kelvin scale and the everyman possible temperature, chosen absolute zero.
Guillaume Amontons was the first to empirically plant the relationship betwixt the pressure and the temperature of a gas (~1700), and Joseph Louis Gay-Lussac determined the relationship more than precisely (~1800). Because of this, the P–T human relationship for gases is known as either Amontons's police or Gay-Lussac's law. Under either proper name, it states that the pressure of a given amount of gas is directly proportional to its temperature on the kelvin calibration when the book is held constant. Mathematically, this tin be written:
[latex]P\propto T\text{ or }P=\text{constant}\times T\text{ or }P=thousand\times T[/latex]
where ∝ ways "is proportional to," and k is a proportionality constant that depends on the identity, amount, and book of the gas.
For a confined, constant book of gas, the ratio [latex]\dfrac{P}{T}[/latex] is therefore constant (i.e., [latex]\dfrac{P}{T}=k[/latex] ). If the gas is initially in "Condition 1" (with [latex]P=P_{1}\text{ and }T = T_{1})[/latex], and then changes to "Condition two" (with [latex]P=P_{2}\text{ and }T = T_{two})[/latex], we have that [latex]\dfrac{{P}_{i}}{{T}_{i}}=k[/latex] and [latex]\dfrac{{P}_{2}}{{T}_{2}}=yard,[/latex] which reduces to [latex]\dfrac{{P}_{i}}{{T}_{ane}}=\dfrac{{P}_{2}}{{T}_{2}}[/latex]. This equation is useful for pressure-temperature calculations for a bars gas at abiding volume. Note that temperatures must be on the kelvin scale for any gas law calculations (0 on the kelvin scale and the lowest possible temperature is called absolute zero). (Also note that there are at least three ways we can depict how the pressure of a gas changes as its temperature changes: We can apply a table of values, a graph, or a mathematical equation.)
Example 1: Predicting Alter in Pressure with Temperature
A tin can of hair spray is used until it is empty except for the propellant, isobutane gas.
- On the can is the alert "Store only at temperatures beneath 120 °F (48.8 °C). Do not incinerate." Why?
- The gas in the can is initially at 24 °C and 360 kPa, and the can has a volume of 350 mL. If the tin can is left in a car that reaches fifty °C on a hot day, what is the new pressure in the can?
Check Your Learning
A sample of nitrogen, N2, occupies 45.0 mL at 27 °C and 600 torr. What pressure will it have if cooled to –73 °C while the book remains constant?
Volume and Temperature: Charles's Law
If we fill a balloon with air and seal it, the balloon contains a specific amount of air at atmospheric pressure, let's say 1 atm. If we put the balloon in a fridge, the gas within gets cold and the airship shrinks (although both the amount of gas and its force per unit area remain constant). If we make the airship very cold, information technology volition shrink a bully deal, and information technology expands again when it warms up.
This video shows how cooling and heating a gas causes its volume to subtract or increase, respectively.
Yous can view the transcript for "Liquid Nitrogen Experiments: The Balloon" here (opens in new window).
These examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are truthful in general: The volume increases as the temperature increases, and decreases as the temperature decreases. Volume-temperature data for a one-mole sample of methane gas at one atm are listed and graphed in Figure four.

Figure 4. The volume and temperature are linearly related for 1 mole of methane gas at a abiding pressure of i atm. If the temperature is in kelvin, volume and temperature are directly proportional. The line stops at 111 K because methane liquefies at this temperature; when extrapolated, it intersects the graph's origin, representing a temperature of absolute cipher.
The human relationship between the volume and temperature of a given amount of gas at constant pressure level is known equally Charles's law in recognition of the French scientist and balloon flight pioneer Jacques Alexandre César Charles. Charles's law states that the volume of a given amount of gas is directly proportional to its temperature on the kelvin calibration when the pressure level is held constant.
Mathematically, this tin can be written as:
[latex]V\propto T\qquad\text{or}\qquad{V}=\text{abiding}\cdot T\qquad\text{or}\qquad{Five}=k\cdot T\qquad\text{or}\qquad{Five}_{1}\text{/}{T}_{1}={V}_{two}\text{/}{T}_{two}[/latex]
with 1000 existence a proportionality constant that depends on the corporeality and force per unit area of the gas.
For a confined, constant pressure level gas sample, [latex]\dfrac{V}{T}[/latex] is constant (i.e., the ratio = k), and as seen with the [latex]V-T[/latex] relationship, this leads to another form of Charles'southward constabulary: [latex]\dfrac{{V}_{1}}{{T}_{1}}=\dfrac{{V}_{2}}{{T}_{ii}}.[/latex]
Case 2: Predicting Modify in Volume with Temperature
A sample of carbon dioxide, CO2, occupies 0.300 L at ten °C and 750 torr. What volume will the gas have at 30 °C and 750 torr?
Check Your Learning
A sample of oxygen, O2, occupies 32.two mL at 30 °C and 452 torr. What volume will it occupy at –seventy °C and the same pressure?
Example three: Measuring Temperature with a Book Modify
Temperature is sometimes measured with a gas thermometer past observing the alter in the book of the gas as the temperature changes at constant pressure level. The hydrogen in a particular hydrogen gas thermometer has a volume of 150.0 cm3 when immersed in a mixture of ice and water (0.00 °C). When immersed in boiling liquid ammonia, the volume of the hydrogen, at the same pressure, is 131.7 cm3. Notice the temperature of boiling ammonia on the kelvin and Celsius scales.
Check Your Learning
What is the volume of a sample of ethane at 467 K and 1.1 atm if information technology occupies 405 mL at 298 One thousand and 1.1 atm?
Volume and Pressure level: Boyle's Police
If nosotros partially fill an airtight syringe with air, the syringe contains a specific amount of air at abiding temperature, say 25 °C. If nosotros slowly push button in the plunger while keeping temperature constant, the gas in the syringe is compressed into a smaller volume and its pressure increases; if we pull out the plunger, the volume increases and the pressure decreases. This example of the effect of book on the pressure of a given amount of a confined gas is true in general. Decreasing the book of a contained gas volition increment its pressure, and increasing its volume will decrease its pressure. In fact, if the volume increases past a certain factor, the pressure level decreases by the same factor, and vice versa. Book-pressure data for an air sample at room temperature are graphed in Figure 5.
Figure 5. When a gas occupies a smaller volume, information technology exerts a college pressure level; when it occupies a larger book, it exerts a lower pressure (assuming the amount of gas and the temperature do not change). Since P and V are inversely proportional, a graph of 1/P vs. V is linear.
Unlike the P–T and V–T relationships, force per unit area and volume are not directly proportional to each other. Instead, P and V showroom inverse proportionality: Increasing the pressure results in a subtract of the book of the gas. Mathematically this can be written:
[latex]P\alpha 1\text{/}5\qquad\text{ or }\qquad{P}=k\cdot 1\text{/}V\qquad\text{ or }\qquad{P}\cdot 5=1000\qquad\text{ or }\qquad{P}_{i}{V}_{1}={P}_{2}{Five}_{2}[/latex]
Effigy 6. The relationship between pressure and volume is inversely proportional. (a) The graph of P vs. Five is a parabola, whereas (b) the graph of (1/P) vs. V is linear.
with k existence a constant. Graphically, this relationship is shown by the direct line that results when plotting the inverse of the pressure [latex]\left(\dfrac{1}{P}\right)[/latex] versus the book (5), or the inverse of book [latex]\left(\dfrac{one}{V}\right)[/latex] versus the pressure (5). Graphs with curved lines are difficult to read accurately at depression or loftier values of the variables, and they are more than hard to apply in plumbing equipment theoretical equations and parameters to experimental information. For those reasons, scientists oftentimes endeavour to find a mode to "linearize" their data. If we plot P versus V, we obtain a hyperbola (see Figure vi).
The relationship between the volume and pressure of a given amount of gas at abiding temperature was beginning published by the English language natural philosopher Robert Boyle over 300 years ago. It is summarized in the argument now known equally Boyle'due south police: The volume of a given corporeality of gas held at abiding temperature is inversely proportional to the pressure nether which it is measured.
Example 4: Volume of a Gas Sample
The sample of gas in Figure v has a volume of 15.0 mL at a pressure of 13.0 psi. Determine the pressure of the gas at a volume of 7.5 mL, using:
- the P–V graph in Figure 5
- the [latex]\dfrac{i}{P}[/latex] vs. 5 graph in Effigy five
- the Boyle's law equation
Comment on the likely accurateness of each method.
Bank check Your Learning
The sample of gas in Figure 5 has a volume of xxx.0 mL at a pressure of six.5 psi. Determine the volume of the gas at a pressure of xi.0 mL, using:
- the P–V graph in Figure 5
- the [latex]\dfrac{i}{P}[/latex] vs. V graph in Effigy 5
- the Boyle's law equation
Comment on the likely accuracy of each method.
Chemical science in Action: Breathing and Boyle's Law
What do yous do near 20 times per minute for your whole life, without interruption, and frequently without even being enlightened of it? The answer, of course, is respiration, or animate. How does it work? It turns out that the gas laws use hither. Your lungs take in gas that your body needs (oxygen) and get rid of waste product gas (carbon dioxide). Lungs are made of spongy, stretchy tissue that expands and contracts while you breathe. When you inhale, your diaphragm and intercostal muscles (the muscles between your ribs) contract, expanding your chest cavity and making your lung volume larger. The increase in book leads to a decrease in pressure (Boyle'southward police). This causes air to flow into the lungs (from high pressure to depression pressure). When you exhale, the process reverses: Your diaphragm and rib muscles relax, your chest cavity contracts, and your lung book decreases, causing the pressure to increment (Boyle's law over again), and air flows out of the lungs (from loftier pressure to low pressure level). You so breathe in and out again, and again, repeating this Boyle's law cycle for the rest of your life (Figure vii).
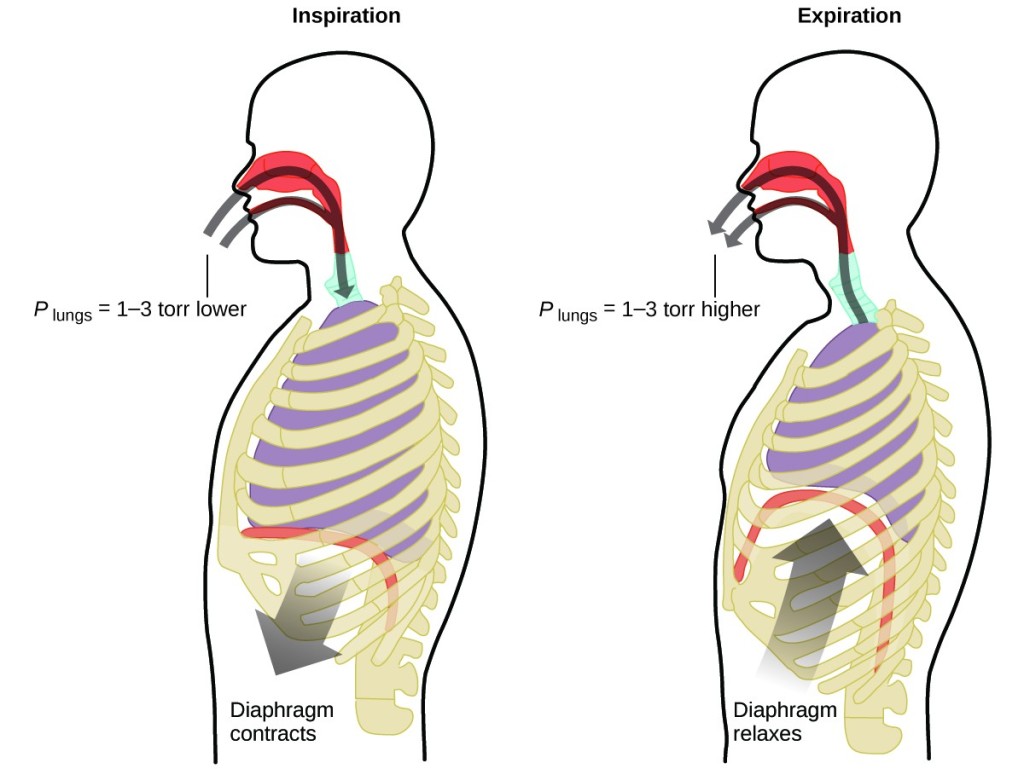
Figure seven. Breathing occurs considering expanding and contracting lung volume creates pocket-sized pressure differences betwixt your lungs and your environment, causing air to exist drawn into and forced out of your lungs.
Moles of Gas and Book: Avogadro's Law
The Italian scientist Amedeo Avogadro advanced a hypothesis in 1811 to account for the beliefs of gases, stating that equal volumes of all gases, measured under the aforementioned weather condition of temperature and pressure, contain the same number of molecules. Over time, this relationship was supported past many experimental observations as expressed by Avogadro's law: For a confined gas, the book (V) and number of moles (northward) are direct proportional if the pressure and temperature both remain constant.
In equation form, this is written as:
[latex]V\propto{northward}\qquad\text{or}\qquad{V}=1000\times{n}\qquad\text{or}\qquad\dfrac{{V}_{1}}{{n}_{1}}=\dfrac{{V}_{2}}{{n}_{2}}[/latex]
Mathematical relationships can also be determined for the other variable pairs, such equally [latex]P[/latex] versus [latex]n[/latex], and [latex]north[/latex] versus [latex]T[/latex].
Visit this interactive PhET simulation link to investigate the relationships between pressure, volume, temperature. and amount of gas. Utilise the simulation to examine the effect of changing one parameter on another while holding the other parameters constant (as described in the preceding sections on the various gas laws).
The Ideal Gas Police force
To this indicate, four separate laws have been discussed that relate pressure, book, temperature, and the number of moles of the gas:
- Boyle'southward police: [latex]PV =[/latex] constant at constant [latex]T[/latex] and [latex]n[/latex]
- Amontons's constabulary: [latex]\dfrac{P}{T}[/latex] = abiding at constant V and n
- Charles'south law: [latex]\dfrac{V}{T}[/latex] = abiding at constant P and northward
- Avogadro's law: [latex]\dfrac{V}{north}[/latex] = abiding at constant P and T
Combining these four laws yields the ideal gas law, a relation between the force per unit area, volume, temperature, and number of moles of a gas:
[latex]PV=nRT[/latex]
where P is the pressure of a gas, V is its volume, n is the number of moles of the gas, T is its temperature on the kelvin scale, and R is a constant called the ideal gas constant or the universal gas constant. The units used to express force per unit area, book, and temperature volition determine the proper class of the gas constant as required by dimensional assay, the most commonly encountered values being 0.08206 L atm mol–1 K–1 and 8.314 kPa L mol–one M–i.
Gases whose properties of P, V, and T are accurately described by the ideal gas law (or the other gas laws) are said to showroom ideal beliefs or to approximate the traits of an ideal gas. An platonic gas is a hypothetical construct that may exist used along with kinetic molecular theory to effectively explain the gas laws as will be described in a later module of this chapter. Although all the calculations presented in this module assume ideal behavior, this assumption is only reasonable for gases nether conditions of relatively low pressure and high temperature. In the final module of this chapter, a modified gas law will exist introduced that accounts for the non-ideal behavior observed for many gases at relatively loftier pressures and low temperatures.
The platonic gas equation contains five terms, the gas constant R and the variable properties P, V, northward, and T. Specifying any four of these terms will permit use of the ideal gas law to calculate the 5th term as demonstrated in the post-obit example exercises.
Example 5: Using the Ideal Gas Law
Methane, CH4, is existence considered for use as an culling automotive fuel to replace gasoline. One gallon of gasoline could be replaced by 655 g of CHiv. What is the book of this much methane at 25 °C and 745 torr?
Check Your Learning
Summate the pressure in bar of 2520 moles of hydrogen gas stored at 27 °C in the 180-L storage tank of a modernistic hydrogen-powered car.
If the number of moles of an platonic gas are kept constant under 2 different sets of conditions, a useful mathematical relationship chosen the combined gas police is obtained: [latex]\dfrac{{P}_{1}{Five}_{i}}{{T}_{1}}=\dfrac{{P}_{2}{V}_{2}}{{T}_{2}}[/latex] using units of atm, L, and One thousand. Both sets of conditions are equal to the product of north × R (where n = the number of moles of the gas and R is the ideal gas law constant).
Case half dozen: Using the Combined Gas Law

Effigy viii. Scuba divers use compressed air to breathe while underwater. (credit: modification of work by Mark Goodchild)
When filled with air, a typical scuba tank with a volume of 13.2 L has a pressure of 153 atm (Figure 8). If the water temperature is 27 °C, how many liters of air will such a tank provide to a diver'south lungs at a depth of approximately 70 feet in the ocean where the force per unit area is 3.13 atm?
Bank check Your Learning
A sample of ammonia is plant to occupy 0.250 50 under laboratory weather condition of 27 °C and 0.850 atm. Find the volume of this sample at 0 °C and 1.00 atm.
The Interdependence between Ocean Depth and Pressure in Scuba Diving

Figure 9. Scuba defined, whether at the Groovy Barrier Reef or in the Caribbean, must exist aware of buoyancy, pressure equalization, and the corporeality of fourth dimension they spend underwater, to avert the risks associated with pressurized gases in the body. (credit: Kyle Taylor)
Whether scuba diving at the Nifty Bulwark Reef in Commonwealth of australia (shown in Figure 9) or in the Caribbean area, divers must sympathize how pressure level affects a number of issues related to their comfort and prophylactic.
Pressure increases with ocean depth, and the pressure level changes most rapidly as divers reach the surface. The pressure a diver experiences is the sum of all pressures above the diver (from the water and the air). About pressure measurements are given in units of atmospheres, expressed every bit "atmospheres absolute" or ATA in the diving customs: Every 33 feet of salt water represents one ATA of pressure in addition to 1 ATA of pressure level from the temper at sea level.
Every bit a diver descends, the increase in pressure causes the torso's air pockets in the ears and lungs to compress; on the rise, the decrease in pressure causes these air pockets to aggrandize, potentially rupturing eardrums or bursting the lungs. Defined must therefore undergo equalization by adding air to torso airspaces on the descent by breathing unremarkably and adding air to the mask by breathing out of the nose or adding air to the ears and sinuses past equalization techniques; the corollary is also true on rising, divers must release air from the torso to maintain equalization.
Buoyancy, or the ability to control whether a diver sinks or floats, is controlled by the buoyancy compensator (BCD). If a diver is ascending, the air in his BCD expands because of lower force per unit area according to Boyle's law (decreasing the pressure of gases increases the volume). The expanding air increases the buoyancy of the diver, and she or he begins to ascend. The diver must vent air from the BCD or risk an uncontrolled ascension that could rupture the lungs. In descending, the increased pressure causes the air in the BCD to compress and the diver sinks much more speedily; the diver must add air to the BCD or gamble an uncontrolled descent, facing much higher pressures near the ocean floor.
The pressure also impacts how long a diver tin stay underwater before ascending. The deeper a diver dives, the more compressed the air that is breathed because of increased pressure level: If a diver dives 33 anxiety, the pressure is 2 ATA and the air would be compressed to one-half of its original volume. The diver uses up available air twice as fast equally at the surface.
Standard Conditions of Temperature and Pressure
We have seen that the volume of a given quantity of gas and the number of molecules (moles) in a given volume of gas vary with changes in pressure and temperature. Chemists sometimes make comparisons against a standard temperature and pressure (STP) for reporting properties of gases: 273.15 K and 1 atm (101.325 kPa). At STP, an ideal gas has a volume of about 22.4 L—this is referred to as the standard tooth volume (Figure 10).
Figure 10. Since the number of moles in a given volume of gas varies with pressure and temperature changes, chemists use standard temperature and pressure level (273.xv Thou and i atm or 101.325 kPa) to study backdrop of gases.
Key Concepts and Summary
The behavior of gases can be described by several laws based on experimental observations of their properties. The pressure level of a given corporeality of gas is directly proportional to its absolute temperature, provided that the volume does not alter (Amontons'south law). The volume of a given gas sample is directly proportional to its absolute temperature at constant force per unit area (Charles's police force). The volume of a given amount of gas is inversely proportional to its pressure when temperature is held abiding (Boyle'south law). Under the same conditions of temperature and pressure, equal volumes of all gases comprise the aforementioned number of molecules (Avogadro'south law).
The equations describing these laws are special cases of the ideal gas police, [latex]PV=nRT[/latex], where P is the pressure of the gas, V is its book, due north is the number of moles of the gas, T is its kelvin temperature, and R is the platonic (universal) gas constant.
Key Equations
- [latex]PV=nRT[/latex]
Try It
- Sometimes leaving a bicycle in the sun on a hot day will cause a blowout. Why?
- Explicate how the volume of the bubbling exhausted past a scuba diver (Figure 8) alter equally they ascent to the surface, assuming that they remain intact.
- One style to state Boyle's constabulary is "All other things beingness equal, the pressure level of a gas is inversely proportional to its volume."
- What is the meaning of the term "inversely proportional?"
- What are the "other things" that must be equal?
- An alternate way to land Avogadro'south law is "All other things being equal, the number of molecules in a gas is directly proportional to the book of the gas."
- What is the meaning of the term "directly proportional?"
- What are the "other things" that must be equal?
- How would the graph in Figure 4 change if the number of moles of gas in the sample used to determine the bend were doubled?
- How would the graph in Figure five change if the number of moles of gas in the sample used to determine the bend were doubled?
- In addition to the data plant in Figure v, what other information do we need to discover the mass of the sample of air used to decide the graph?
- Decide the volume of 1 mol of CHiv gas at 150 K and i atm, using Figure four.
- Make up one's mind the pressure of the gas in the syringe shown in Figure v when its volume is 12.5 mL, using:
- the appropriate graph
- Boyle'south law
- A spray tin can is used until it is empty except for the propellant gas, which has a pressure of 1344 torr at 23 °C. If the can is thrown into a fire (T = 475 °C), what will be the pressure in the hot tin can?
- What is the temperature of an 11.2-50 sample of carbon monoxide, CO, at 744 torr if information technology occupies 13.three L at 55 °C and 744 torr?
- A ii.50-L volume of hydrogen measured at –196 °C is warmed to 100 °C. Summate the volume of the gas at the higher temperature, bold no alter in pressure.
- A balloon inflated with three breaths of air has a book of 1.vii L. At the aforementioned temperature and force per unit area, what is the book of the airship if v more same-sized breaths are added to the balloon?
- A weather condition balloon contains 8.80 moles of helium at a pressure of 0.992 atm and a temperature of 25 °C at ground level. What is the volume of the balloon nether these atmospheric condition?
- The book of an automobile air bag was 66.8 Fifty when inflated at 25 °C with 77.eight g of nitrogen gas. What was the force per unit area in the bag in kPa?
- How many moles of gaseous boron trifluoride, BF3, are contained in a 4.3410-50 bulb at 788.0 K if the force per unit area is 1.220 atm? How many grams of BFthree?
- Iodine, I2, is a solid at room temperature simply sublimes (converts from a solid into a gas) when warmed. What is the temperature in a 73.3-mL bulb that contains 0.292 g of Itwo vapor at a pressure level of 0.462 atm?
- How many grams of gas are present in each of the following cases?
- 0.100 L of CO2 at 307 torr and 26 °C
- eight.75 L of C2Hiv, at 378.3 kPa and 483 Grand
- 221 mL of Ar at 0.23 torr and –54 °C
- A loftier distance balloon is filled with i.41 × 104 50 of hydrogen at a temperature of 21 °C and a pressure level of 745 torr. What is the book of the balloon at a height of 20 km, where the temperature is –48 °C and the pressure is 63.1 torr?
- A cylinder of medical oxygen has a volume of 35.iv L, and contains Oii at a pressure of 151 atm and a temperature of 25 °C. What volume of O2 does this correspond to at normal trunk conditions, that is, 1 atm and 37 °C?
- A large scuba tank (Figure eight) with a volume of xviii 50 is rated for a force per unit area of 220 bar. The tank is filled at 20 °C and contains plenty air to supply 1860 L of air to a diver at a pressure level of 2.37 atm (a depth of 45 feet). Was the tank filled to chapters at 20 °C?
- A 20.0-L cylinder containing 11.34 kg of butane, C4H10, was opened to the atmosphere. Calculate the mass of the gas remaining in the cylinder if it were opened and the gas escaped until the pressure in the cylinder was equal to the atmospheric force per unit area, 0.983 atm, and a temperature of 27 °C.
- While resting, the average 70-kg human male consumes 14 L of pure O2 per hr at 25 °C and 100 kPa. How many moles of O2 are consumed by a 70 kg human being while resting for 1.0 h?
- For a given amount of gas showing ideal behavior, draw labeled graphs of:
- the variation of P with V
- the variation of V with T
- the variation of P with T
- the variation of [latex]\dfrac{1}{P}[/latex] with V
- A liter of methane gas, CH4, at STP contains more than atoms of hydrogen than does a liter of pure hydrogen gas, H2, at STP. Using Avogadro's law as a starting signal, explicate why.
- The effect of chlorofluorocarbons (such as CCl2F2) on the depletion of the ozone layer is well known. The use of substitutes, such as CH3CH2F(g), for the chlorofluorocarbons, has largely corrected the trouble. Calculate the volume occupied by 10.0 g of each of these compounds at STP:
- CCltwoFtwo(thousand)
- CH3CH2F(g)
- As 1 thou of the radioactive element radium decays over one twelvemonth, it produces i.16 × 1018 blastoff particles (helium nuclei). Each alpha particle becomes an atom of helium gas. What is the pressure level in pascal of the helium gas produced if information technology occupies a book of 125 mL at a temperature of 25 °C?
- A balloon that is 100.21 Fifty at 21 °C and 0.981 atm is released and just barely clears the height of Mount Crumpet in British Columbia. If the final volume of the balloon is 144.53 50 at a temperature of v.24 °C, what is the pressure experienced past the balloon as information technology clears Mount Crumpet?
- If the temperature of a fixed amount of a gas is doubled at constant volume, what happens to the pressure?
- If the book of a stock-still corporeality of a gas is tripled at constant temperature, what happens to the pressure?
Glossary
absolute zero:temperature at which the volume of a gas would be zero according to Charles's law.
Amontons's police force:(also, Gay-Lussac'due south police force) pressure of a given number of moles of gas is directly proportional to its kelvin temperature when the volume is held abiding
Avogadro's police force:volume of a gas at abiding temperature and pressure is proportional to the number of gas molecules
Boyle'southward police:book of a given number of moles of gas held at abiding temperature is inversely proportional to the pressure under which information technology is measured
Charles's law:volume of a given number of moles of gas is directly proportional to its kelvin temperature when the pressure is held constant
ideal gas:hypothetical gas whose physical properties are perfectly described past the gas laws
ideal gas constant (R):abiding derived from the ideal gas equation R = 0.08226 L atm mol–one K–1 or eight.314 L kPa mol–1 G–ane
platonic gas police:relation between the pressure, volume, amount, and temperature of a gas under weather derived by combination of the simple gas laws
standard weather condition of temperature and pressure (STP):273.15 K (0 °C) and 1 atm (101.325 kPa)
standard molar volume:book of i mole of gas at STP, approximately 22.iv Fifty for gases behaving ideally
ligertwoodwhoustatich.blogspot.com
Source: https://courses.lumenlearning.com/chemistryformajors/chapter/relating-pressure-volume-amount-and-temperature-the-ideal-gas-law/